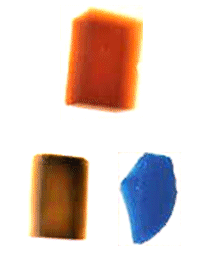Metal Oxide Aerogels
Metal oxide aerogels are the inorganic cousins of the more common silica aerogel–each type with its own unique properties. These aerogels are important as they can act as catalysts for various chemical transformations, matrices for explosives, precursors for other materials (such as carbon nanotube catalysts), can be magnetic, and are often quite colorful.
Up until the 1990’s, metal oxide aerogels had been historically much more difficult to synthesize than silica aerogels, largely because there were no good synthetic routes for making metal oxide gels. This is mostly due to the difficulty associated with handling metal alkoxide compounds (as they hydrolyze readily), or in some cases simply because of there aren’t any or any good metal alkoxides for a particular metal.
A Note About Metal Oxide Naming
In chemistry, many metal oxides are referred to by changing the “-ium” suffix of the metal to “-ia”. Thus, aluminum oxide is often called alumina, chromium oxide is often called chromia, zirconium oxide is often called zirconia, etc. METAL OXIDES ARE NOT METALS. This is a common misunderstanding among the general public and a frequent oversight in the media. Just as rust is a form of iron oxide (which doesn’t have a special name, incidentally) and is very different from iron metal, or how silica (glass) is very different from silicon (a semiconductor), metal oxides are chemically different from their parent metals.
Metal Oxides Make Colorful Aerogels
One of the most notable differences between metal oxide aerogels and silica aerogels is that many metal oxide aerogels are often brilliantly colored. It is important to remember that the bluish cast characteristic of a silica aerogel is a result of Rayleigh scattering by nanoparticles which make up the aerogel backbone, and that silica itself is not blue. Similarly, aerogels of metal oxides which are white as powders and clear in their crystalline form (such as alumina, titania , and zirconia among others) look like silica aerogels–transparent with bluish Rayleigh scattering, and perhaps occasionally somewhat cloudy white. However, many metal oxides (such as chromium oxide and iron oxide) exhibit bright coloring as both powders and crystals, and are in fact used in dyes, paints, and glazes because of this. As a result, aerogels of these oxides are also brightly colored–iron oxide aerogels are Martian red, for example. Below are some of the colors associated with different metal oxide aerogels.
Silica, Alumina, Titania, Zirconia: Clear with Rayleigh scaterring blue or white
Iron Oxide: Rust red or yellow, opaque
Chromia: Deep green or deep blue, opque
Vandia: Olive green, opaque
Neodymium Oxide: Purple, transparent
Samarium Oxide: Yellow, transparent
Holmium Oxide, Erbium Oxide: Pink, transparent
Old School Metal Oxide Aerogel Preparation
Although Samuel Kistler is reported to have prepared aerogels of tin oxide in the 1930’s, the next metal oxide aerogels wouldn’t pop up until the 1980’s. These were the first alumina aerogels, first synthesized by Dr. Bulant Yoldas through the controlled hydrolysis of aluminum tri-sec-butoxide–a viscous, difficult-to-handle aluminum alkoxide with the consistency of honey. Suffice it to say this technique has its limitations and produced alumina aerogels of limited quality as a result. Aerogels of titania, zirconia, hafnia, vanadia, niobia, and tantala were eventually prepared using alkoxides as well, however unlike silica, good monolithicity and high optical transparency were hard to achieve in these aerogels.
In general, metal oxide aerogels can be prepared by carefully hydrolyzing a respective metal alkoxide in a solvent. Because of the increased sensitivity of most metal alkoxides over silicon alkoxides, often times very dry solvents and tightly controlled amounts of water must be used, and often times also cryogenic temperatures to slow the reaction rates down to a productive speed. Schematically the process proceeds similar to silica alkoxide sol-gel chemistry, in that alkoxide groups are hydrolyzed to hydroxyl groups, and then hydroxyl groups from two molecules condense, resulting in a metal-oxygen-metal bridge. This proceeds, resulting in the formation of a metal oxide sol which can then crosslink into a metal oxide gel. The gels can then purified, aged, and supercritically dried similar to other gels. Generally speaking, though, the quality of metal oxide gels made through alkoxide chemistry is generally poor (mechanically and optically speaking).
The alkoxide method is the preferred method for producing aerogels of vanadia and molybdenum oxide, for which it works quite well. The opaque olive green vanadia aerogels have a remarkable worm-like nanostructure which give them extremely high strength-to-weight ratios–much higher than other types of aerogels.
The Wonderful World of Epoxide-Assisted Gelation
In the mid-1990’s, Drs. Tom Tillotson, Alex Gash, Joe Satcher, John Poco, Larry Hrubesh, and Randall Simpson at Lawrence Livermore National Laboratory developed a new technique for synthesizing metal oxide gels: epoxide-assisted gelation of metal salts. This technique proved to not only enable preparation of metal oxide gels previously not possible, but also made synthesis of metal oxide gels much easier and resulted in stronger gels (and thus aerogels) with much better monolithicity. Gash further developed the technique in the early 2000’s by identifying the underlying mechanisms and extending it to many new elements. For this reasons the technique is commonly referred to as the “Gash Prep”.
In this technique, a hydrated metal salt (such as a nitrate or a chloride) is dissolved in a solvent (such as water, ethanol, or half-water/half-ethanol), to which an epoxide (such as propylene oxide or epichlorohydrin) is added dropwise. The metal salts exist as some sort of aquo complex in solution (that is, they have water ligands attached to them). The epoxide then acts as a proton scavenger, stealing a proton away from the metal complex and protonates itself, resulting in an irreversible ring-opening reaction. The pH of the system, in turn, gently increases, and aquo-hydroxy metal complexes form. These species, which contain M-OH sites, can then condense together to form metal-oxygen-metal bridges (similar to the way Si-OH groups condense together to form silicon-oxygen-silicon bridges)–the necessary connectivity for producing a metal oxide gel. Upon supercritical drying of the resulting gel, a metal oxide aerogel is produced.
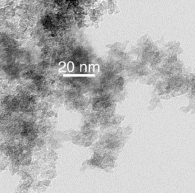
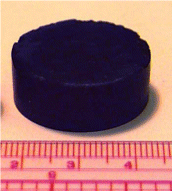
Take the example of a chromia (chromium oxide) aerogel. In this system, chromium(III) nitrate nonahydrate is dissolved in ethanol and propylene oxide is added. Chromium is present as an aquo complex [Cr(H2O)6]3+ in this system, which of course contains protons. The propylene oxide then acts as a proton scavenger, stealing a proton away from this complex and protonates itself. The pH of the system thus increases and aquo-hydroxy species Cr3+ ([Cr(OH)x(H2O)6-x](3-x)+) form. These species, which contain Cr-OH sites, can then condense to form Cr-O-Cr bridges, forming a chromia sol which eventually crosslinks into a chromia gel in about 3 h. Upon supercritical drying, a deep, dark blue chromia aerogel is afforded (seen in the picture with the ruler). Under a transmission electron microscope, a nanostructure very similar to silica aerogels is observed.
As mentioned, this technique is not limited to just chromium salts, and works with a wide variety of metal salts, preferably nitrates and chlorides. Alumina, titania, zirconia, hafnia, niobia, tantala, tungsten oxide, iron oxide, indium oxide, gallium oxide, and tin oxide aerogels can readily be prepared with this technique, to name a few. Using this technique, Drs. Jeffrey Long and Debra Rolison of the United States Naval Research Laboratory have demonstrated preparation of magnetic iron oxide aerogels.
In 2001, Livermore developed high-strength alumina aerogels with epoxide-assisted gelation which exhibit a fascinating acicular (leaf-like) morphology. Alumina aerogels have some important potential benefits over silica aerogels, including a higher melting point and the ability to be partially crystallized. Dr. Ted Baumann at Livermore has developed the technique for alumina aerogels further since then.
Lanthanide and Actinide Oxide Aerogels
Of course, the underappreciated f-block elements can be coaxed into metal oxide aerogel frameworks through epoxide-assisted gelation as well. Lanthana and thoria aerogels have been reported. Nanocrystalline thoria aerogels, which are radioactive, are of interested for nuclear systems, as well as catalysts for electrodes in batteries and fuel cells. Assumedly, aerogels of urania and plutonia can be prepared too, however no reports of these materials are available.
Difficult-to-Prepare Metal Oxide Aerogels
Unfortunately, not all metal oxides can be made into aerogels easily, and for some metals, it may not be possible at all. Molybdenum oxide aerogels were elusive for quite some time, since molybdenum tends to form terminal Mo=O oxo bonds as opposed to Mo-O-Mo bridges. Without a macroscopic network of Mo-O-Mo bridges, a molybdenum oxide gel cannot form, and only a sol of unconnected nanoparticles or a precipitate will result. Molybdenum oxide aerogels were fiinally prepared in 1998 by Drs. Winny Dong and Bruce Dunn at the University of California–Los Angeles from molybdenum isopropoxide by performing a ligand exchange with acetonitrile (CH3CN) to replace some of the isopropoxide ligands with acetonitrile ligands. Hydrolysis of this new complex results in the formation of both Mo=O and Mo-O-Mo bonds, with Mo-O-Mo bonds favored enough that a gel can form. With a synthetic route to molybdenum oxide gels available, molybdenum oxide aerogels could be prepared. Since epoxide-assisted gelation of molybdenum(VI) chloride does not result in a gel (or at least is not straightforward), this remains as the only synthetic route to molybdenum oxide aerogels. As of now, vanadia and manganese oxide aerogels also need to be prepared from alkoxide precursors as opposed to epoxide-assisted gelation of their metal salts as well.
Metal salts in which the metal center is in an oxidation state of less than 3+ do not form gels with epoxide-assisted gelation easily, especially the late (right-side) third-row transition metals.. Only recently have nickel oxide and copper oxide aerogels been prepared, and only very recently (2007) have zinc oxide aerogels been prepared by Prof. Louisa Hope-Weeks at Texas Tech University. Additionally, no synthetic route has been established for preparing aerogels of alkali oxides (Li2O, Na2O, K2O, Rb2O, Cs2O) or alkaline earth oxides (MgO, CaO, SrO, BaO, RaO). In all of these cases, it’s not hard to imagine that the reason no aerogels have been prepared is because a continuous expanse of metal-oxygen-metal network bonding is hard to form, since there are only one or two bonds to be made, and a three-dimensional structure cannot result. Furthermore, there are no reports of aerogels of noble metal oxides (the noble metals being Pd, Rh, Ir, and Pt), largely due to difficulties arising from their square-planar configurations and because they don’t readily form oxides.
Although there are no reports of rhenium oxide or osmium oxide aerogels in the literature, Dr. Debra Rolison at the Naval Research Laboratory claims to have prepared these in the past.
Properties of Metal Oxide Aerogels
See the Properties of Aerogels page for an overview of the materials properties of various metal oxide aerogels.