How is Aerogel Made?
So how exactly do you make an aerogel?
As described in the What is Aerogel? section, an aerogel is the intact, dry, ultralow density, porous solid framework of a gel (that is, the part that gives a gel its solid-like cohesiveness) isolated from the gel’s liquid component (which takes up most of the volume in the gel). But how do you isolate such a material from a gel?
The Start of an Aerogel: A Gel
Aerogels start their life out as a gel, physically similar to Jell-O®. A gel is a colloidal system in which a nanostructured network of interconnected particles spans the volume of a liquid medium. Gels have some properties like liquids, such as density, and some properties like solids, such as a fixed shape. In the case of Jell-O, this network of particles is composed of proteins and spans the volume of some sort of fruit juice. A gel is structurally similar to a wet kitchen sponge, only with pores a thousand to a million times smaller. Because a gel’s pores are so small, the capillary forces exerted by the liquid are strong enough to hold it inside the gel and prevent the liquid from simply flowing out. It’s important to remember that gelatin isn’t the only type of gel–in fact, chemists can prepare gels with backbones composed of many organic and inorganic substances and many liquid interiors.
Once a gel is prepared, it must be purified prior to further processing. This is because the chemical reactions that result in the formation of a gel leave behind impurities throughout the gel’s liquid interior that interfere with the drying processes used to prepare aerogel (as described below). Purification is done by simply soaking the gel under a pure solvent (depending on the gel this could be acetone, ethanol, acetonitrile, etc.), allowing impurities to diffuse out and pure solvent to diffuse in. The solvent in which the gel is soaked is typically exchanged with fresh solvent multiple times over the course several days. Depending on the volume and geometry of the gel, diffusive processes can take any where from hours to weeks. A ice-cube size sample can usually be purified in 1 or 2 days.
For more information about gels and gel preparation, see The Sol-Gel Process under The Science of Aerogel.
The Dire Consequences of Evaporatively Drying a Gel
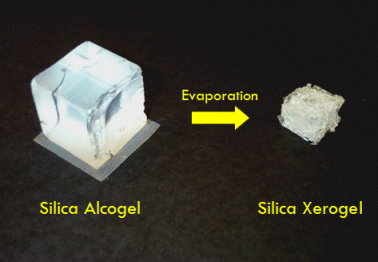
Now, if you’ve ever left Jell-O uneaten and uncovered in the refrigerator for a long while (on the order of a week or so), you may have observed the gel shrinks gradually. This occurs when the liquid trapped in the gel evaporates from the gel’s surface. As molecules of liquid escape into the air, the surrounding liquid molecules are pulled together by capillary action and tug on the framework of the gel. Continued evaporation results in collapse of the framework of the gel, forming a dense, hard substance with less than 10% of the volume of the original gel. This is called xerogel (pronounced zeroGEL). In fact, 1980’s-style hard contact lenses used to be manufactured by drying silica gels into lens-shaped silica xerogels.
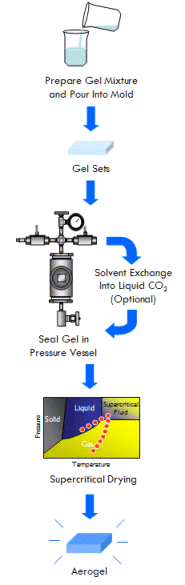
Aerogel is the solid framework of a gel isolated from its liquid component, prepared in such a way as to preserve the framework’s pore structure (or at least most of it). In other words, aerogel is what would be left over if you could remove the liquid from a gel without causing it to shrink. This is most effectively done through a special technique called supercritical drying (although as you will see below, there are other ways to make aerogel as well).
The Answer: Supercritical Drying
In general, supercritical drying is used when liquid needs to be removed from a sample that would be damaged by evaporative or other drying techniques. Biological specimens, for example, are often preserved through supercritical drying.
Supercritical drying is a clever technique by which we can pull the rug out from under capillary action (so to speak). As mentioned earlier, capillary action induced by liquid evaporating from a gel’s pores causes the gel to shrink. So what if there were some way to avoid capillary forces to begin with? This is where supercritical drying comes in.
All pure substances (that won’t decompose) have what’s called a critical point–a specific and characteristic pressure and temperature at which the distinction between liquid and gas disappears. For most substances, the critical point lies at a fairly high pressure (>70 atmospheres) and temperature (>400°F). At the critical point, the liquid and vapor phases of a substance merge into a single phase that exhibits the behavior of a gas (in that it expands to fill the volume of its container and can be compressed) but simultaneously possesses the density and thermal conductivity of a liquid. This phase is called a supercritical fluid.
Say we have a sealed container containing a liquid below its critical point inside and equipped with a pressure gauge on top. In fact, a certain amount of liquid will evaporate in the container until the vapor pressure of the liquid is reached in the container, after which no more liquid will evaporate and the gauge will read a corresponding stable pressure. Now if we heat this container, we will notice the pressure in the container increases, since the vapor pressure of a liquid increases with increasing temperature. As the critical point draws near, the pressure in the container squeezes molecules in the vapor close enough together that the vapor becomes almost as dense as a liquid. At the same time, the temperature in the container gets high enough that the kinetic energy of the molecules in the liquid overwhelms the attractive forces that hold them together as a liquid. In short, as the pressure and temperature in the container get closer to the critical point, the liquid phase becomes more gas-like and the vapor phase more liquid-like. Finally, the critical point is reached and the meniscus dividing the two phases blurs away, resulting in a single supercritical phase. As this occurs, the surface tension in the fluid gradually drops to zero, and thus the ability of the fluid to exert capillary stress does too.
Aerogelification
In the case of making aerogels, a gel is placed in a pressure vessel under a volume of the same liquid held within its pores (lets say ethanol for example). The pressure vessel is then slowly heated to the liquid’s critical temperature. As this happens, the vapor pressure of the liquid increases, causing the pressure in the vessel to increase and approach the critical pressure of the liquid. The critical point is then surpassed, gently transforming the liquid in the gel (as well as the liquid and vapor surrounding the gel) into a supercritical fluid. Once this happens, the ability of the fluid in the gel to exert capillary stress on the gel’s solid framework structure of the gel has decreased to zero.
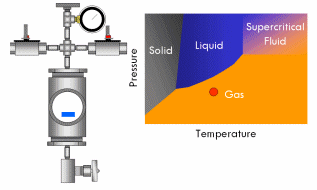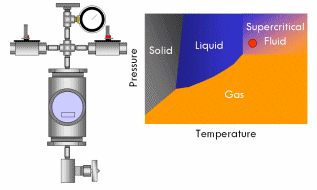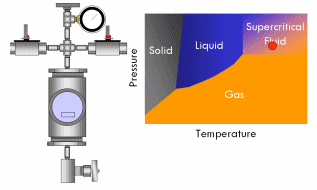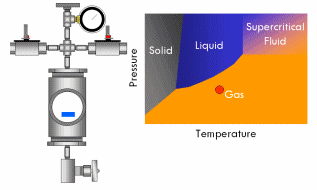 With supercritical fluid now present throughout the entire vessel and permeating the pores of the gel, the fluid in the gel can be removed. This is done by partially depressurizing the vessel, but not so much as to cause the pressure in the vessel to drop below the critical pressure. The temperature of the vessel must also remain above the critical temperature during this step. The goal is to remove enough fluid from the vessel while the fluid is still supercritical so that when the vessel is fully depressurized/cooled down and drops below the fluid’s critical point, there will simply not be enough substance left in the vessel left for liquid to recondense. This might require several cycles of heating (and thus pressurizing) followed by depressurization (again all done above the critical point). Once enough fluid has been removed from the vessel, the vessel is slowly depressurized and cooled back to ambient conditions. As this happens, the fluid in the vessel passes back through the critical point, but since much of the fluid has been removed and the temperature is still elevated as the vessel depressurizes, the fluid reverts to a gas phase instead of a liquid phase. What was liquid in the gel has been converted into a gas without capillary stress every arising, and an aerogel is left behind.
With supercritical fluid now present throughout the entire vessel and permeating the pores of the gel, the fluid in the gel can be removed. This is done by partially depressurizing the vessel, but not so much as to cause the pressure in the vessel to drop below the critical pressure. The temperature of the vessel must also remain above the critical temperature during this step. The goal is to remove enough fluid from the vessel while the fluid is still supercritical so that when the vessel is fully depressurized/cooled down and drops below the fluid’s critical point, there will simply not be enough substance left in the vessel left for liquid to recondense. This might require several cycles of heating (and thus pressurizing) followed by depressurization (again all done above the critical point). Once enough fluid has been removed from the vessel, the vessel is slowly depressurized and cooled back to ambient conditions. As this happens, the fluid in the vessel passes back through the critical point, but since much of the fluid has been removed and the temperature is still elevated as the vessel depressurizes, the fluid reverts to a gas phase instead of a liquid phase. What was liquid in the gel has been converted into a gas without capillary stress every arising, and an aerogel is left behind.
It is important to note, however, that most of the liquids used in the preparation of gels are organic solvents such as methanol, ethanol, acetone, and acetonitrile, and such liquids are potentially dangerous at the temperatures and pressures required to make them supercritical. To make the aerogelification process less dangerous, the liquid component of a gel can be exchanged with a non-flammable solvent that mixes well with organic solvents–liquid carbon dioxide (see below).
For more information about supercritical drying, see The Science of Aerogel section.
The Hunt Process: Making Supercritical Drying Safer With Liquid Carbon Dioxide
In the early 1980’s, Dr. Arlon Hunt at Lawrence Berkeley National Laboratory developed a technique for preparing aerogels without needing to supercritically extract potentially explosive solvents. In this technique, a gel containing an organic solvent (such as methanol, ethanol, acetone, or acetonitrile) is soaked under liquid carbon dioxide to replace the liquid in the gel with liquid CO2. CO2, which is the product of combustion reactions, is inherently non-flammable (since it’s already oxidized), and has a low critical point of only 31.13°C (88.03°F) and 7.375 MPa (1069.7 psi, or 72.786 times atmospheric pressure). This is compared with, say, methanol, which is very flammable and has a critical point of 239.5°C (463.1°F) and 8.084 MPa (1172.5 psi, 79.783 times atmospheric pressure).
One drawback, however, is that unlike methanol or other organic solvents, CO2 does not exist as a liquid at ambient conditions. In fact, dry ice, the solid form of CO2 (which you can buy at some gas stations and grocery stores), sublimes directly to gaseous CO2 at atmospheric pressure instead of melting. As a result, in order to work with liquid CO2 so that we can soak a gel in it, we have to use CO2 at a pressure where it can exist as a liquid (around 58 times atmospheric pressure at room temperature). This doesn’t really pose much of a problem, though, since we need to do the supercritical extraction in a pressure vessel eventually anyway.
To perform CO2 exchange, a gel is placed in a pressure vessel which is then sealed and slowly pressurized with a tank of liquid CO2 equipped with a siphon tube (like a liquid soap dispenser). Liquid CO2 siphon tanks are common, and can be found in almost any restaurant or bar as the source of carbonation in a soda fountain system. Liquid CO2 entering the vessel will boil instantly, pressurizing the vessel until the vapor pressure of liquid CO2 at room temperature (~58 atm) is reached. At that point, liquid CO2 will siphon into the vessel and cover the gel. Depending on the size of the vessel and the gel, it is common to pre-fill the vessel with organic solvent (whatever is in the gel) to prevent the gel from drying out while waiting for CO2 to siphon in. This organic solvent is then drained off as soon as CO2 starts to siphon in. After liquid CO2 has siphoned in, the gel is simply allowed to soak for a number of hours. The liquid in the vessel is drained out and replaced with new liquid every few hours for a period of time of 1-3 days for small samples and up to a week or two for large samples. As mentioned, liquid CO2 doesn’t exist at ambient conditions, so when liquid CO2 is drained from a pressurized vessel, although the liquid level goes down in the vessel, only gaseous CO2 and churnings of dry ice evolve from the drain valve.
As the gel soaks in the liquid CO2, the organic solvent held within its pores diffuses out, and liquid CO2 diffuses in its place. Once the gel has been thoroughly diffused through with CO2 (and this is up to the researcher’s discretion), supercritical extraction can be performed just as described above.
Learn how to build a supercritical dryer of your own and find a fully-illustrated step-by-step process of performing supercritical drying with CO2 under the Make section.
How Big Can an Aerogel Be Made?
Just as you can only bake a pie as big as your oven, you can only supercritically dry an aerogel as large as your pressure vessel. This means one of three things–either you need a big supercritical dryer, you limit yourself to making small aerogels, or you use a non-supercritical drying technique (see below). Additionally, large continuous volumes (such as cubes or spheres) are generally difficult to make since it takes exponentially longer for solvent from the interior of the gel to diffuse out of the gel as the gel thickness is increased. However, hollow cubes and spheres, flat plates and discs, and rods with thicknesses less than two inches (5 cm), regardless of how big the gel’s other dimensions are, can be easily made.
This said, there are many techniques for preparing aerogel materials called ambigels (often just referred to as aerogels) with subcritical drying techniques. These materials typically have porosities of 50-95% and so they are usually (but not always) a little less dense than supercritically-dried aerogels. Subcritical drying techniques typically require specially-modified gels, in which the solid framework of the gel is chemically changed so that liquid is less able to stick to it and thus exerts only minimal stress on the gel upon evaporation. Additionally, the liquid in the pores of the gel is frequently replaced with a liquid that has a low surface tension, such as pentane or hexane, so that when the liquid is evaporated little capillary stress can result. Cabot Corp.’s Nanogel® aerogel granules are made through a subcritical drying technique.
See Aerogel Without the Pressure under The Science of Aerogel section for more information.